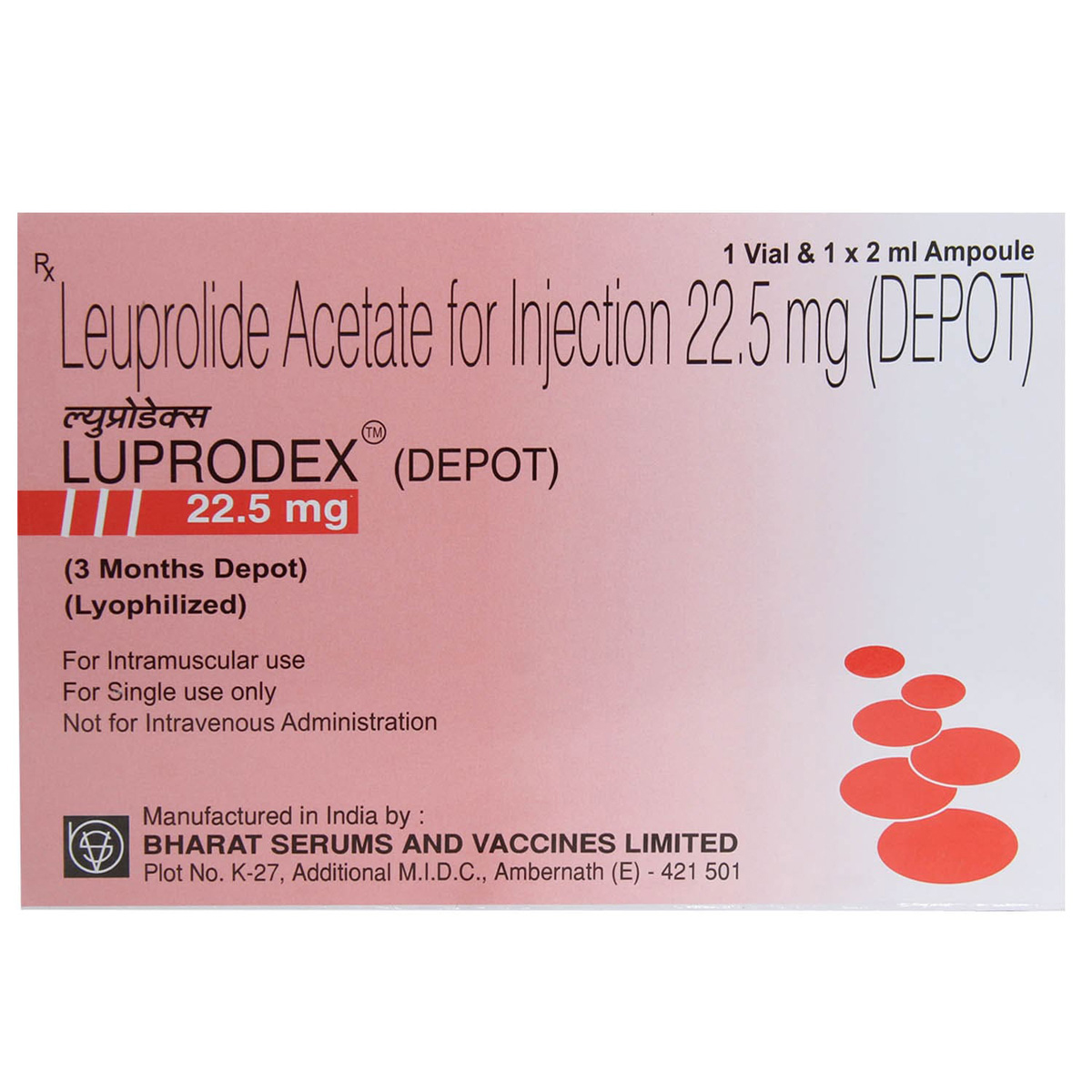
Leuprosta Depot 22.5 mg Injection
MRP ₹15995.5
(Inclusive of all Taxes)
₹1919.5 Cashback (12%)
know your delivery time
Provide Delivery Location
Composition :
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :

Secure Payment

Trusted by 8 Crore Indians

Genuine Products
Therapeutic Class
Country of origin
Manufacturer/Marketer address
Author Details
We provide you with authentic, trustworthy and relevant information
FAQs
Leuprosta Depot 22.5 mg Injection contains Leuprolide, a synthetic hormone that helps reduce the testosterone and oestrogen hormones in men and women, respectively. This process of lowering hormone levels can decrease cancer cell's growth in prostate cancer in men and shrinks endometriosis in women.
Yes, Leuprosta Depot 22.5 mg Injection commonly affects the hair by making them thin which further causes loss of hair. However, it is not very common. Hair reduction possibly happens due to the estrogen lowering effect of Leuprosta Depot 22.5 mg Injection. These effects are not long-lasting and may return after some time. If it matters to you then inform your doctor for further advice.
Leuprosta Depot 22.5 mg Injection may cause impotence and affect fertility in men. It is advised to speak to your doctor if you plan to have a family in the future before starting Leuprosta Depot 22.5 mg Injection.
Leuprosta Depot 22.5 mg Injection should be used with caution in diabetic patients as it may increase the risk of high blood sugar levels. Therefore, regular monitoring of blood sugar levels is recommended while taking Leuprosta Depot 22.5 mg Injection and inform your doctor if you have diabetes before taking Leuprosta Depot 22.5 mg Injection.
Leuprosta Depot 22.5 mg Injection may worsen your mood disorders like depression. Hence it is advised to use Leuprosta Depot 22.5 mg Injection only when you are free from any kind of mental problems. Please consult your doctor for more information.
Leuprosta Depot 22.5 mg Injection is not recommended for patients suffering from heart rhythm problems such as arrhythmia (irregular heartbeat) as it may increase the risk of heart rhythm problems. Therefore, inform your doctor if you have any heart problems before taking Leuprosta Depot 22.5 mg Injection.
Leuprosta Depot 22.5 mg Injection may cause osteoporosis (bone thinning) due to the reduction of estrogen in the body. Regular bone density tests may help you monitor osteoporosis. Contact your doctor if you observe any sign of tendon pain or swelling while taking Leuprosta Depot 22.5 mg Injection.
Disclaimer
Alcohol
Safe if prescribed
You are recommended to avoid alcohol consumption while taking Leuprosta Depot 22.5 mg Injection to avoid unpleasant side-effects. Alcohol intake, along with Leuprosta Depot 22.5 mg Injection, may cause increased drowsiness.
Pregnancy
Consult your doctor
Leuprosta Depot 22.5 mg Injection is a pregnancy category X drug. Leuprosta Depot 22.5 mg Injection should not be used during pregnancy because it causes harm to the fetus (newborn baby). Both women of childbearing potential and men must use an effective method of contraception while taking fluorouracil and for at least 6 months afterwards. Please consult your doctor about any concerns regarding this.
Breast Feeding
Consult your doctor
Leuprosta Depot 22.5 mg Injection should not be taken during breastfeeding as it passes into the breastmilk and may harm the nursing baby. It is contraindicated in breastfeeding mothers.
Driving
Safe if prescribed
Leuprosta Depot 22.5 mg Injection causes dizziness and affects your ability to drive. Do not drive or operate machinery if you experience any unmanageable side effects with Leuprosta Depot 22.5 mg Injection.
Liver
Consult your doctor
If you have had a history or evidence of any liver-related diseases, please consult the doctor before taking Leuprosta Depot 22.5 mg Injection. Your doctor will weigh the benefits and any potential risks before prescribing it to you.
Kidney
Consult your doctor
If you have had a history or evidence of any kidney-related diseases, please consult the doctor before taking Leuprosta Depot 22.5 mg Injection. Your doctor will weigh the benefits and any potential risks before prescribing it to you.
Children
Safe if prescribed
Leuprosta Depot 22.5 mg Injection is not recommended in children younger than two years of age.
Product Substitutes
Keep Refrigerated. Do not freeze.
About Leuprosta Depot 22.5 mg Injection
Leuprosta Depot 22.5 mg Injection belongs to the class of 'anticancer or anti-neoplastic agents', primarily used to treat prostate cancer in men, breast cancer and endometriosis in women and precocious puberty (early puberty) in children. Prostate cancer is the prostate gland's cancer (a small gland under the bladder secretes fluid that nourishes and protects sperm) found only in men. Breast cancer is a type of cancer that develops in breast cells stimulated by a female sex hormone called oestrogen. Endometriosis is a disorder in which tissue lining the uterus grows outside the uterine cavity. Precocious puberty occurs when a child's body changes into that of an adult or experiences puberty too soon.
Leuprosta Depot 22.5 mg Injection contains Leuprolide that belongs to the class of gonadotropin-releasing hormone (GnRH) agonists. It is a synthetic hormone that acts similar to the GnRH, produced by the hypothalamus gland in the brain. It works by inhibiting the synthesis of the natural male hormone, testosterone in men and oestrogen in women. This process of lowering hormone levels can decrease cancer cell's growth in prostate cancer in men and shrinks endometriosis and breast cancer in women.
Take Leuprosta Depot 22.5 mg Injection as prescribed by your doctor. Your doctor will decide the dosage depending on your medical condition. The common side effects of Leuprosta Depot 22.5 mg Injection include hot flashes, decreased sexual interest, shrinking of the testicles, breast tenderness or swelling, dizziness, headache, nausea, vomiting, diarrhoea, upset stomach and injection site pain reaction. These side effects are not familiar to everyone and vary individually. If you notice any side effects that are not manageable, please consult your doctor.
To treat your condition effectually continue taking Leuprosta Depot 22.5 mg Injection for as long as your doctor has prescribed. Do not stop Leuprosta Depot 22.5 mg Injection midway. Avoid taking Leuprosta Depot 22.5 mg Injection if you are pregnant or breastfeeding because this Leuprosta Depot 22.5 mg Injection can cause harmful effects on the unborn baby. Both women and men using this Leuprosta Depot 22.5 mg Injection should use birth control to avoid pregnancy. Leuprosta Depot 22.5 mg Injection may also affect fertility in men, hence discuss with your doctor if you have any plans to start a family in the future. Leuprosta Depot 22.5 mg Injection is not recommended for children below two years of age. Leuprosta Depot 22.5 mg Injection may make you feel dizzy, hence drive when you are alert only.
Uses of Leuprosta Depot 22.5 mg Injection
Medicinal Benefits Mweb
Key Benefits
Leuprosta Depot 22.5 mg Injection contains Leuprolide, a gonadotropin-releasing hormone (GnRH) agonist. It treats prostate cancer in men, endometriosis in women and precocious puberty (early puberty) in children. Leuprosta Depot 22.5 mg Injection is a synthetic hormone that acts similar to the GnRH, produced by the hypothalamus gland in the brain. Being an anti-neoplastic or anticancer agent, Leuprosta Depot 22.5 mg Injection works by lowering testosterone levels in men that help stop the growth of cancer cells in prostate cancer. Leuprosta Depot 22.5 mg Injection reduces oestrogen (a hormone required for the development and regulation of the female reproductive system and secondary sexual characteristics) levels in women, thus helps in shrinking of endometriosis and breast cancer.
Directions for Use
Side Effects of Leuprosta Depot 22.5 mg Injection
- Weight changes
- Hot flushes
- Muscle weakness
- Bone Pain
- Loss of interest in sexual intercourse
- Inability to have an erection
- Tiredness
- Difficulty sleeping
- Headaches
- Breast tenderness
- Changes in breast size
- Mood swings
- Abdominal pain
- Vomiting (feeling sick)
- Acne
- Vaginal Bleeding
- Spotting
- Injection site reactions (skin hardening, redness, pain, abscesses, swelling, nodules, ulcers and skin damage)
Drug Warnings
If you are allergic to Leuprosta Depot 22.5 mg Injection or any other medicines, please tell your doctor. Leuprosta Depot 22.5 mg Injection can affect the unborn baby when used in pregnancy. Hence, Leuprosta Depot 22.5 mg Injection is not indicated for use in pregnancy and breastfeeding women. If you are using Leuprosta Depot 22.5 mg Injection, make sure to use reliable forms of contraception to prevent pregnancy during the course. Leuprosta Depot 22.5 mg Injection may also affect fertility in men, hence discuss with your doctor if you have any plans to start a family in the future. Use the Leuprosta Depot 22.5 mg Injection with caution in elderly patients and children. Leuprosta Depot 22.5 mg Injection is not recommended for children younger than two years of age. Your symptoms may become temporarily worse when you first start taking Leuprosta Depot 22.5 mg Injection. Inform your doctor if this continues for longer than 2 months. If you have a seizure or unusual changes in mood or behaviour, inform your doctor immediately. Before starting Leuprosta Depot 22.5 mg Injection, let your doctor know if you have any medical history of liver or kidney diseases, heart diseases, recent heart attack, depression, brain tumours, electrolyte imbalance, diabetes, fits, weak bones and high cholesterol levels. Leuprosta Depot 22.5 mg Injection may cause QT prolongation (heart muscle takes longer than normal to recharge between beats) and may affect heart rhythm, hence heart patients should be cautious while taking Leuprosta Depot 22.5 mg Injection. Leuprosta Depot 22.5 mg Injection can make you feel dizzy and affects your mental ability to drive. Do not drive or operate machinery if you are not mentally alert and focused.
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Using ibutilide together with leuprolide can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Ibutilide together can possibly result in an interaction, they can be taken together if advised by your doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Taking Leuprolide and Eliglustat together can raise the risk of an abnormal heart rhythm that may be serious.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Eliglustat together can possibly result in an interaction, they can be taken together if prescribed by a doctor. However, consult your doctor if you experience any unusual symptoms. Do not discontinue any medications without consulting a doctor.
Taking leuprolide with cisapride can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Although taking Leuprosta Depot 22.5 mg Injection with Cisapride is not recommended, it can be taken if prescribed by a doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or an irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Coadministration of Leuprosta Depot 22.5 mg Injection and citalopram can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection and citalopram together can possibly result in an interaction; they can be taken together if advised by your doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or an irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Taking leuprolide with dofetilide together can raise the risk of an abnormal heart rhythm.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Dofetilide together can possibly result in an interaction, they can be taken together if prescribed by a doctor. However, consult your doctor if you experience sudden dizziness, lightheadedness, fainting, or shortness of breath. Do not discontinue any medications without consulting a doctor.
Taking leuprolide with Saquinavir together can raise the risk of an abnormal heart rhythm.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Saquinavir together can possibly result in an interaction, they can be taken together if advised by your doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Taking Leuprosta Depot 22.5 mg Injection with Vandetanib together can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Vandetanib together can possibly result in an interaction, they can be taken together if advised by your doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Taking Leuprosta Depot 22.5 mg Injection with Pimozide together can raise the risk of an abnormal heart rhythm.
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Pimozide together can possibly result in an interaction, they can be taken together if advised by your doctor. However, contact your doctor if you experience drowsiness, lightheadedness, fainting, shortness of breath, or irregular heartbeat. Do not stop taking any medications without consulting a doctor.
Using nilotinib together with leuprolide can increase the risk of an irregular heart rhythm that may be serious
How to manage the interaction:
Taking Leuprosta Depot 22.5 mg Injection with Nilotinib is not recommended, as it can result in an interaction. It can be taken if your doctor has suggested it. However, if you experience prolonged diarrhea, vomiting, dizziness, lightheadedness, fainting, or difficulty breathing, contact your doctor immediately. Do not stop using any medications without talking to your doctor.
Taking Leuprosta Depot 22.5 mg Injection and Bedaquiline can increase the risk of an irregular heart rhythm.
How to manage the interaction:
Although taking Leuprosta Depot 22.5 mg Injection and Bedaquiline together can evidently cause an interaction, it can be taken if your doctor has suggested it. However, if you experience dizziness, fainting, irregular heart rhythm, or difficulty breathing, it's important to consult your doctor immediately. Do not stop using any medications without a doctor's advice.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Drug-Diseases Interactions
Drug-Diseases Interactions
Login/Sign Up
Drug-Drug Interactions Checker List
- QUINIDINE
- PROCAINAMIDE
- DISOPYRAMIDE
- AMIODARONE
- DEXAMETHASONE
- METHYLPREDNISOLONE
- PREDNISOLONE
- MOXIFLOXACIN
- METHADONE
- CITALOPRAM
- ESCITALOPRAM
- FLUOXETINE
- FLUVOXAMINE
- PAROXETINE
- SERTRALINE
Habit Forming
Special Advise
- Your doctor may advise liver function tests to monitor your liver health since Leuprosta Depot 22.5 mg Injection may cause increased liver enzymes (alanine aminotransferase, serum aspartate and serum transaminases).
- Leuprosta Depot 22.5 mg Injection may cause increased serum glucose levels (hyperglycaemia), hence inform your doctor if you have diabetes before starting Leuprosta Depot 22.5 mg Injection.
- Your doctor may advise blood tests, electrolyte levels and regular examinations of the prostate.
- It is advised to get an electrocardiogram (ECG) done timely to monitor your heart health.
Diet & Lifestyle Advise
- Take the medication as directed by the doctor and at regular intervals.
- Maintain a fibre-rich diet and include healthy carbohydrates from fruits, vegetables and whole grains.
- Include fish, soy, tomatoes, Brussels sprouts, kale, broccoli and oils containing omega-3 fatty acids such as olive oil as these foods may reduce the risk of prostate cancer.
- Avoid grilled meat, red meat, saturated fat found in animal products, milk and dairy products.
- Exercise regularly to lose weight as obesity is also considered a risk factor for prostate cancer.
- Eat at regular intervals.
- Keep your weight under control with BMI 19.5-24.9.
- Opt for a diet rich in whole grains, fruits, veggies and low-fat dairy products.
All Substitutes & Brand Comparisons
RX
Out of StockLeprol 22.5 mg Injection 1's
Aureate Healthcare
₹12984
(₹10646.9 per unit)
24% CHEAPERRX
Out of StockLurpova 22.5mg Injection
₹13800
(₹11316.0 per unit)
19% CHEAPERRX
Glerelin 22.5 mg Injection 1's
Glenmark Pharmaceuticals Ltd
₹13981
(₹12582.9 per unit)
10% CHEAPER

Have a query?
Buy best Neoplastic Disorders products by
Intas Pharmaceuticals Ltd
Natco Pharma Ltd
Dr Reddy's Laboratories Ltd
Cipla Ltd
Celon Laboratories Pvt Ltd
Sun Pharmaceutical Industries Ltd
Alkem Laboratories Ltd
United Biotech Pvt Ltd
Zydus Cadila
Zydus Healthcare Ltd
Neon Laboratories Ltd
Glenmark Pharmaceuticals Ltd
Mylan Pharmaceuticals Pvt Ltd
BDR Pharmaceuticals Internationals Pvt Ltd
Emcure Pharmaceuticals Ltd
Adley Formulations
Samarth Life Sciences Pvt Ltd
Hetero Drugs Ltd
Torrent Pharmaceuticals Ltd
Fresenius Kabi India Pvt Ltd
Pfizer Ltd
Admac Lifesciences(Oncology)
Adley Pharmaceuticals Ltd
Novartis India Ltd
Halsted Pharma Pvt Ltd
Getwell Life Sciences India Pvt Ltd
Lupin Ltd
Cadila Healthcare Ltd
Hetero Healthcare Pvt Ltd
GLS Pharma Ltd
Reliance Formulation Pvt Ltd
Abbott India Ltd
Aimcad Biotech Pvt Ltd
Astra Zeneca Pharma India Ltd
Therdose Pharma Pvt Ltd
Axiommax Oncology Pvt Ltd
Biochem Pharmaceutical Industries Ltd
Khandelwal Laboratories Pvt Ltd
Msn Laboratories Pvt Ltd
Aureate Healthcare
Caitlin Oncology
Dabur India Ltd
Zee Laboratories Ltd
Sarabhai Chemicals (India) Pvt Ltd
Wembrace Biopharma Pvt Ltd
Delarc Pharmaceuticals Pvt Ltd
Medaegis Biotek Pvt Ltd
Panacea Biotec Ltd
RPG Life Sciences Ltd
Shilpa Medicare Ltd
Getwell Oncology Pvt Ltd
Ipca Laboratories Ltd
Miracalus Pharma Pvt Ltd
Biocon Ltd
Cadila Pharmaceuticals Ltd
Eli Lilly and Company (India) Pvt Ltd
Ferring Pharmaceuticals Pvt Ltd
Fresenius Kabi Oncology Ltd
Getwell Pharmaceutical Pvt Ltd
Maximal Healthcare Pvt Ltd
Boehringer Ingelheim India Pvt Ltd
Del Trade International Pvt Ltd
Sayre Therapeutics Pvt Ltd
Akumentis Healthcare Ltd
Corona Remedies Pvt Ltd
Eisai Pharmaceuticals India Pvt Ltd
GlaxoSmithKline Pharmaceuticals Ltd
Hilfen Pharmaceuticals Pvt Ltd
Johnson & Johnson Pvt Ltd
Mankind Pharma Pvt Ltd
Merck Ltd
Oncostar Pharma Pvt Ltd
Rhone Poulenc Rorer India Pvt Ltd
Vhb Life Sciences Inc
Zuventus Healthcare Ltd
Admac Pharma Ltd
Adonis Laboratories Pvt Ltd
Alniche Life Sciences Pvt Ltd
BPRL Pvt Ltd
Caitlin Life Care
Dr Care
German Remedies Ltd
Janssen Pharmaceuticals Pvt Ltd
MEDICAMEN BIOTECH LTD
Maxis Healthcare (I) Pvt Ltd
Pharm Products Pvt Ltd
Accord Life Spec Pvt Ltd
Amps Biotech Biotech Pvt Ltd
Aphia Healthcare
Astellas Pharma India Pvt Ltd
Bangalore Pharmaceutical and Research Laboratory Pvt Ltd (BPRL)
Bharat Serums and Vaccines Ltd
Cellgen Biopharma
Cosmic Life Sciences
Daris Biocare
East West Pharma India Pvt Ltd
Hillrock Biotech Pvt Ltd
Lucien Life Sciences
Maneesh Pharmaceuticals Ltd
Medilief Bioscience Pvt Ltd