Typbar TCV Vaccine
MRP ₹2353
(Inclusive of all Taxes)
₹352.9 Cashback (15%)
know your delivery time
Provide Delivery Location
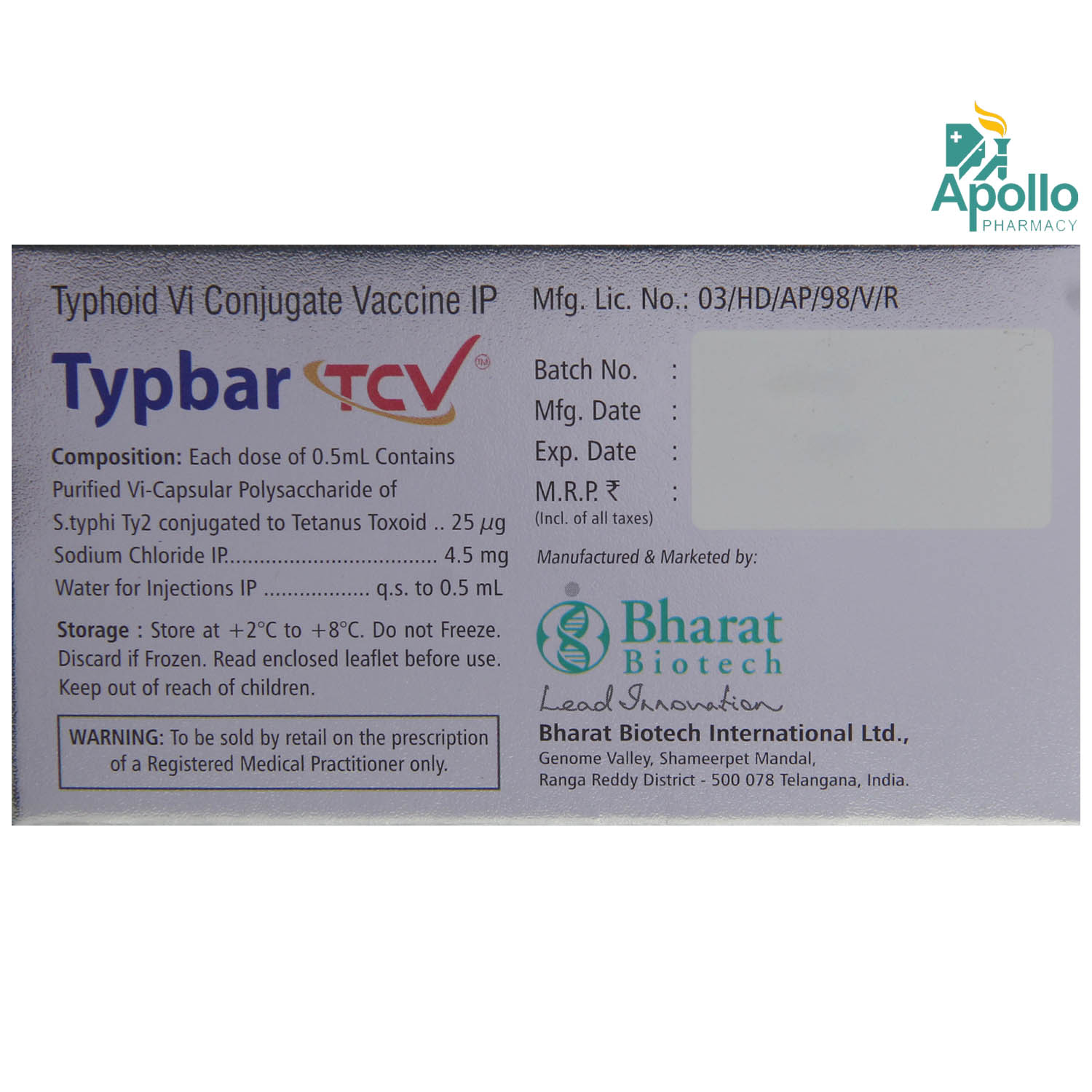
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :

Secure Payment

Trusted by 8 Crore Indians

Genuine Products
Therapeutic Class
Country of origin
Manufacturer/Marketer address
FAQs
Typbar TCV Vaccine works by stimulating antibody production and by mounting an immune response in the body. Thereby preventing typhoid.
A headache could be a side effect of Typbar TCV Vaccine . Although not everyone experiences this side effect if it persists or worsens consult a doctor.
There is not enough literature to explain the effects of Typbar TCV Vaccine on pregnancy. Typbar TCV Vaccine should be used in pregnancy only if absolutely warranted. If absolutely needed, vaccination with Typbar TCV Vaccine during pregnancy should be delayed until the second or third trimester to reduce the risks of foetal harm.
Typbar TCV Vaccine offers high-rate protection against typhoid, and the immunity may last up to 2-3 years across all age groups.
Typbar TCV Vaccine is essential for people who may travel to places where there is a risk of exposure to typhoid fever.
Disclaimer
Alcohol
Safe if prescribed
Exercise caution while using Typbar TCV Vaccine with alcohol.
Pregnancy
Consult your doctor
Typbar TCV Vaccine is not recommended during pregnancy unless absolutely warranted. Please consult your doctor if you are pregnant.
Breast Feeding
Consult your doctor
It is unknown whether Typbar TCV Vaccine is excreted in human milk. Typbar TCV Vaccine is given to breastfeeding mothers only if the doctor thinks benefits are greater than risks. Therefore please consult a doctor if you are breastfeeding.
Driving
Safe if prescribed
Typbar TCV Vaccine may cause tiredness in some people. Avoid driving if you feel tired after taking Typbar TCV Vaccine .
Liver
Consult your doctor
If you have any concerns regarding the use of Typbar TCV Vaccine in patients with liver problems, please consult a doctor.
Kidney
Consult your doctor
If you have any concerns regarding the use of Typbar TCV Vaccine in patients with kidney problems, please consult a doctor.
Children
Safe if prescribed
Typbar TCV Vaccine is safe for use in children above 2years. The safety and effectiveness of Typbar TCV Vaccine in children below 2years have not been established.
Keep Refrigerated. Do not freeze.Prepaid payment required.
Reference
- https://www.medicines.org.uk/emc/medicine/6186#gref
- https://www.who.int/biologicals/publications/trs/areas/vaccines/typhoid/WHO_TRS_840_A1.pdf
- https://en.wikipedia.org/wiki/Vi_capsular_polysaccharide_vaccine
- https://www.fda.gov/media/75993/download
- https://www.bharatbiotech.com/typbartcv.html
- https://timesofindia.indiatimes.com/life-style/food-news/typhoid-what-to-eat-and-what-to-avoid-when-suffering-from-it/photostory/78519304.cms
- https://pubmed.ncbi.nlm.nih.gov/12441674/
- https://www.cdc.gov/vaccines/hcp/vis/vis-statements/typhoid.html
- https://www.bharatbiotech.com/images/typbartcv/Typbar-TCV-Package-Insert.pdf
- https://www.healthline.com/nutrition/typhoid-diet#foods-to-eat-avoid
- https://www.mayoclinic.org/diseases-conditions/typhoid-fever/symptoms-causes/syc-20378661
About Typbar TCV Vaccine
Typbar TCV Vaccine is a vaccine belonging to the group of immunising agents indicated for the active immunization for the prevention of typhoid. Typhoid is an abdominal bacterial infection caused by Salmonella typhi. It is characterised by stomach ache, headache, high fever, abdominal pain, diarrhoea, weakness, and vomiting.
Typbar TCV Vaccine contains Purified Vi Polysaccharide Typhoid Vaccine and Sodium chloride. Typbar TCV Vaccine works by stimulating the body to produce antibodies against the induced capsular component of the bacterium, thereby helping their body to mount an immune response and fight the diseases they have been vaccinated against, in the future.
In some cases, Typbar TCV Vaccine may cause certain common side effects such as headache, fever, redness, pain, and inflammation at the injection site. Most of these side effects do not require medical attention and will resolve gradually over time. However, you are advised to talk to your doctor if you experience these side effects consistently.
Before taking Typbar TCV Vaccine , inform the doctor if you are allergic to any of its components. Consult your doctor if you are pregnant and nursing. Typbar TCV Vaccine may cause tiredness, so be cautious while driving. The safety and effectiveness of Typbar TCV Vaccine in children below 2years have not been established. Keep your doctor informed about your health condition and medications to rule out any interactions.
Uses of Typbar TCV Vaccine
Medicinal Benefits Mweb
Key Benefits
Typbar TCV Vaccine is a vaccine. It contains Purified Vi Polysaccharide Typhoid Vaccine and Sodium chloride. It is used to prevent typhoid disease. Typbar TCV Vaccine induces a non-toxic and safe form of the causative bacteria’s capsular specimen into the body, which stimulates their body to produce antibodies and provides immunity. This immune response is remembered by the body, helping it stay prepared in case of a future invasion by these bacteria.
Directions for Use
Side Effects of Typbar TCV Vaccine
- Fever
- Redness and pain at injection site
- Headache
- Muscle pain
Drug Warnings
Before taking Typbar TCV Vaccine , inform the doctor if you are allergic to any of its components. Keep the doctor informed if you have any immunocompromised states and metabolic diseases such as HIV, cancer, hypo/agammaglobulinaemia or any chronic disorders. Avoid using Typbar TCV Vaccine in case of a severe infection or very high fever/temperature. Inform your doctor of any immunosuppressant medicines, alkylating agents, antimetabolites, chemotherapy, radiotherapy or any other herbs or vitamins you may be on. Consult your doctor if you are pregnant and nursing.
Drug-Drug Interactions Checker List
- DOXORUBICIN
- CYCLOSPORINE
- TACROLIMUS
- PREDNISOLONE
- MERCAPTOPURINE
- FLUOROURACIL
Habit Forming
Special Advise
- Please inform the doctor immediately if you notice any anaphylaxis (allergy, skin changes, difficulty in breathing, anxiety, confusion, dizziness etc) post-injection.
- To preclude any harm due to fainting (which may be seen rarely), procedures to prevent falling and to prevent any injuries due to falls, should be in place.
- If the vaccine was frozen by accident, discard it.
- If you experience any of these, consult a doctor immediately: a high fever within 2 days post-vaccination, a shock-like state within 2 days of vaccination- persistent crying lasting 3 hours or more within 2 days of vaccination - fits with or without fever within 3 days of vaccination.
Diet & Lifestyle Advise
- If your child is severely ill, it is best to wait before vaccination.
- It is important to keep your child hydrated post-vaccination. Make sure to give them plenty of water.
- In case of fever, consult your doctor before treating with any OTC medication.
- Make your child’s personal hygiene a top priority. Keeping their hands clean and washing them with soap and water regularly is important in preventing infection. Opt for traditional hand washing over sanitisers.
- Avoid unclean water. Make sure the water is clean.
- Ensure you avoid raw fruits and vegetables in your child’s diet as these are breeding grounds for the bacterium. Also, avoid foods served at room temperatures and prefer hot foods.
All Substitutes & Brand Comparisons

Have a query?
Buy best Vaccines products by
Serum Institute Of India Pvt Ltd
GlaxoSmithKline Pharmaceuticals Ltd
Biological E Ltd
Abbott India Ltd
Bharat Biotech
Sanofi India Ltd
Human Biologicals Institute
Msd Pharmaceutical Pvt Ltd
Panacea Biotec Ltd
Zydus Healthcare Ltd
Bharat Sanchar Nigam Ltd
Cadila Healthcare Ltd
Lupin Ltd
Zuventus Healthcare Ltd
Bharat Serums and Vaccines Ltd
Biomed
Indian Immunologiclas Ltd
Novartis India Ltd
Pfizer Ltd
Baxter India Pvt Ltd
Bharath
Bio-Med Pvt Ltd
Biomed Pharma
Cpl Biologicals Pvt Ltd
Dr Reddy's Laboratories Ltd
Indiabulls Pharmaceuticals Pvt Ltd
Indian Drugs & Pharmaceuticals Ltd
Kamada Pharmaceuticals
Mankind Pharma Pvt Ltd
Novamed Pharma
Novo Medi Sciences Pvt Ltd
Ranbaxy Laboratories Ltd
Samarth Life Sciences Pvt Ltd
Shantha Biotech
Smith & Kenner Pharma Pvt Ltd
Synergy Pharmaceuticals
Unison Pharmaceuticals Pvt Ltd
Vins Bio Products Ltd
Wockhardt Ltd
Zydus Cadila

